Lasagna's Law has met its match
The cycle is sadly familiar: Your trial protocol is finalized. You’ve signed on the usual primary investigators (PIs) and sites. But despite their best estimates, when it comes time to recruit patients, they’re nowhere to be found.
To increase enrollment, one option is to add more sites. Another is to amend the protocol. No matter what, your timeline and budget have taken a hit. Competitors have a better shot of beating you to market or further entrenching their positions, and your program spend faces more scrutiny from your leaders or board members.
Dr. Louis Lasagna, founder of what is now known as the Tufts Center for the Study of Drug Development, famously and facetiously identified this patient-finding problem more than 40 years ago with his eponymous law — that our industry is prone to over-estimate the number of patients eligible for a given study.
It’s fair to say that not much has changed because north of 80% of clinical trials in the U.S. today fail to hit their enrollment targets.* While others continue to admire the problem, Optum Life Sciences is doing something about it.
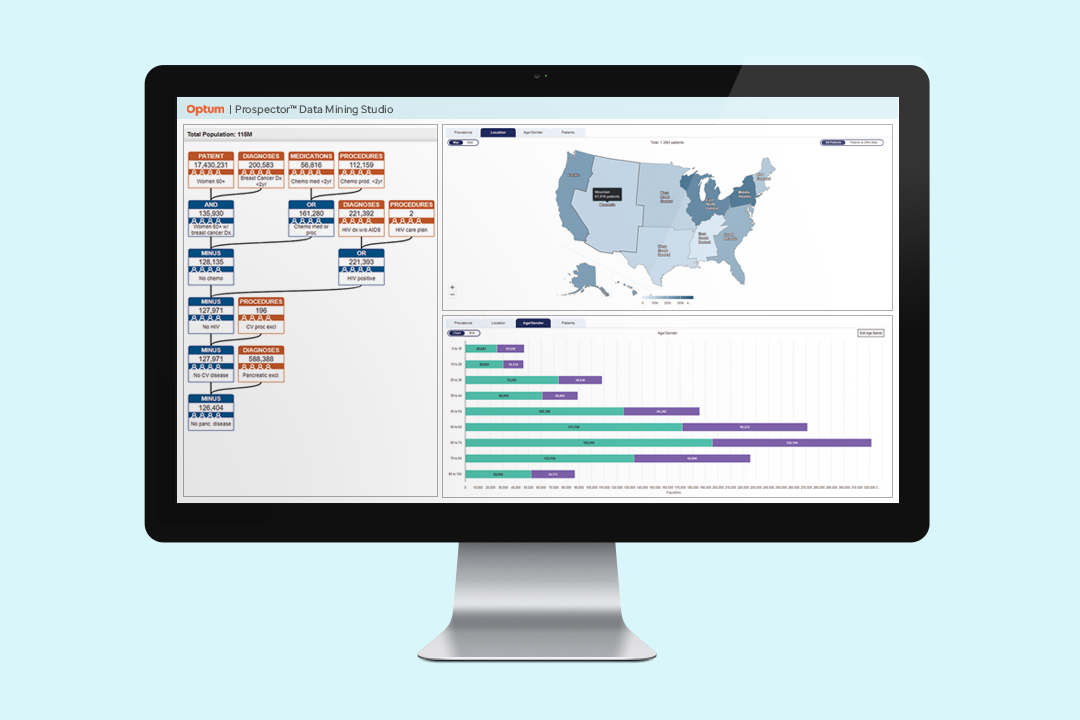
Our Prospector® Data Mining Studio helps clinical development leaders quickly and easily examine study feasibility and optimize protocol development. Prospector is built on the Optum de-identified EHR database, with over 100 million lives of clinical data and years of regulatory-grade and research-grade analysis behind it.
Without any programming skill needed, users can model different study designs and assess changes to a protocol’s inclusion and exclusion criteria, seeing the effects on the available patient population in real-time. Prospector also tallies the number of potentially eligible patients reachable through our Digital Research Network, offering an even faster path to full enrollment.
Armed with the right information, you can confidently design your study with a recruitable population in mind — and finally break the cycle of wasted time, effort and money. Connect with us to schedule a Prospector demo today.
Schedule a demo today
Get a better idea of how your eligibility criteria impact study feasibility, based on clinical data from over 100 million lives.
Insights from Prospector are helping us to go back and check internally if specific criteria are intentional or not, with the goal of avoiding a protocol amendment in the future.
Head of Feasibility, Top 30 Pharma
Designed with you in mind
Check out these key differentiators of the Prospector Data Mining Studio.
Precision anchoring
Apply temporal criteria (such as 'patient prescribed Drug X within 60 days of Diagnosis Y') to get realistic estimates of patient counts.
Data exploration
Examine data at the individual or population level to generate hypotheses, find answers and help improve representation in clinical trials.
Access to NLP concepts and unstructured notes
Query natural language processing (NLP) concepts within the tool itself and work with our team to analyze unstructured notes.
Streamlined patient finding and activation
We can work with our Digital Research Network sites to find protocol-eligible patients and share vetted lists with study coordinators.

Learn more about the Optum clinical data set
Prospector leverages the pan-therapeutic, NLP-enriched Optum de-identified clinical data set, with over 100 million patient lives.
Use cases for the Prospector Data Mining Studio
- Fine-tune protocol criteria to optimize across regulatory, scientific and health equity aims.
- Experiment with how various criteria modifications can boost patient counts and see which factors contribute to patient attrition.
- Rapidly assess overall study feasibility and pinpoint areas of risk.
- Avoid protocol amendments by vetting criteria against a highly representative data set.
- Gather disease insights (market size, clinical characteristics of specific populations, individual patient journeys, etc.) to support market planning and clinical strategy.
- Discover trends that spark ideas for further analysis (by epidemiology teams or health economics and outcomes researchers) to inform value and evidence strategy.
Complementary solutions
Clinical trial solutions
Enhance clinical trial design and performance.
Digital Research Network
Increase efficiency and reduce the burden on site staff.
True Source direct data capture tool
Help improve regulatory-grade data collection accuracy and ease trial site burden.
Related resources

Break down silos in clinical trial design with RWD and tools

Webinar: Solving trial recruitment challenges with data